(通讯员 周浩 魏子晴)大脑的学习和记忆是一种高度协调的精细过程,突触囊泡运载神经递质等化学物质,将突触信息从一个神经元传递给下一个神经元,以此完成神经元之间的信息交流。突触分泌过程中膜融合和相关大分子调控的机制是近年来该领域的热点问题。
突触分泌中膜融合的过程高度依赖SNARE蛋白(Syntaxin-1、SNAP-25和Synaptobrevin-2) 从N端到C端以“拉链”形式组装成反式SNARE复合物,进而将突触囊泡和质膜拉近来完成膜融合。这一过程受到突触前膜生物大分子高度调控。CAPS (Calcium-dependent secretion activator)和Munc13家族蛋白在突触囊泡 (synaptic vesicle,SV) 和致密核心囊泡(dense core vesicle,DCV)的胞吐分泌中起着重要的调控作用。研究表明,Munc13-1蛋白能通过“MUN”功能区激活Syntaxin-1蛋白,进而启动SNARE复合物的组装,从而促进胞吐分泌的发生。相对而言,CAPS-1蛋白在突触分泌中的机制一直不清楚。
3月19日, 8797威尼斯老品牌的马聪教授课题组在《Cell Reports》上在线发表了题为 "Structural and Functional Analysis of the CAPS SNARE-Binding Domain Required for SNARE Complex Formation and Exocytosis" 的研究论文。文章报道了CAPS-1和Munc13-1通过协同调控SNARE复合物组装模式,从而时序性调控突触分泌。
研究发现, CAPS-1蛋白的DAMH功能区(和Munc13-1 MUN功能区存在结构相似性)不具备激活Syntaxin-1蛋白的功能;相反,它通过与Munc13-1 MUN结合,强烈抑制Munc13-1激活Syntaxin-1的能力,这一功能可以在突触分泌初期有效控制Munc13-1活性并保证突触囊泡在突触前膜的有效富集。在Syntaxin-1被激活后,CAPS-1又能持续稳定Syntaxin-1的激活状态,进而加速SNARE复合物的组装, 并最终促进突触分泌过程的发生。
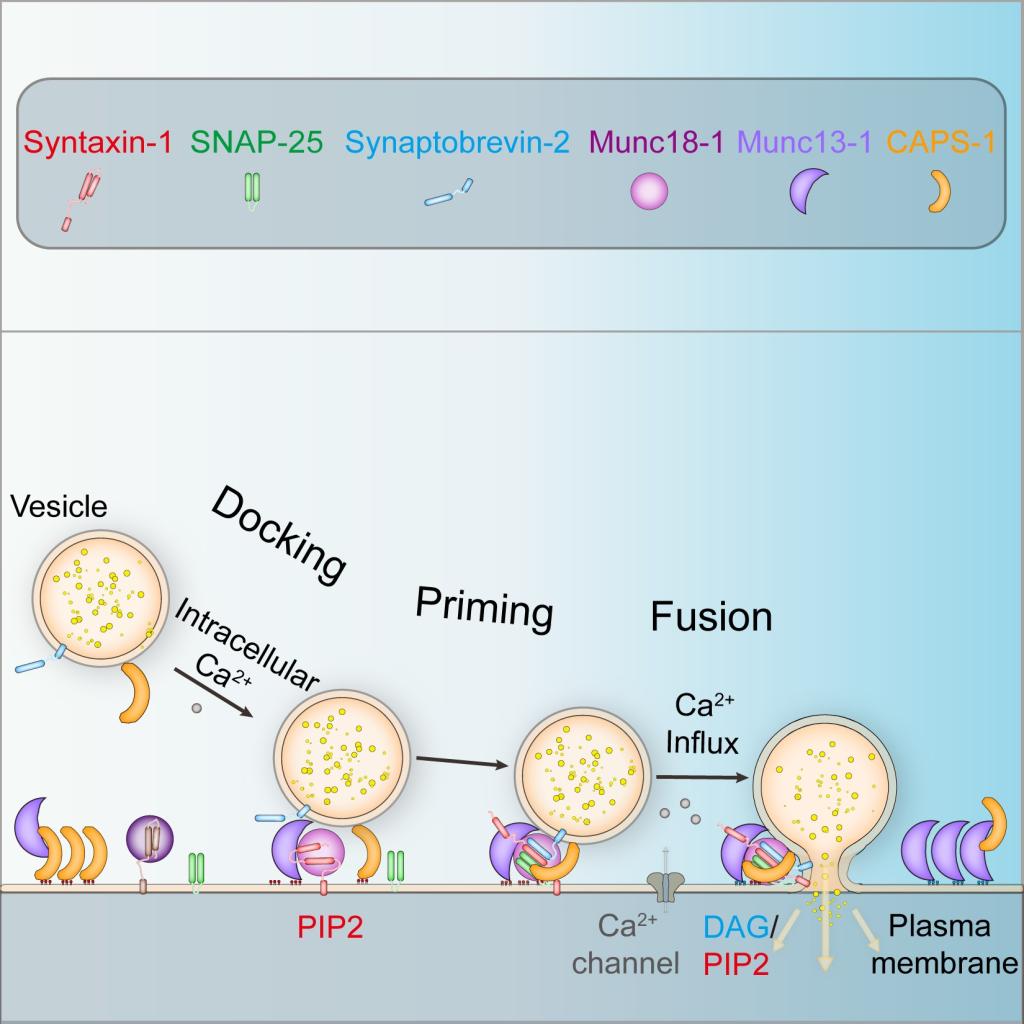
此项研究成果拓展了马聪教授课题组在神经递质分泌机制领域的观点【Science (2013),Nature Structural & Molecular Biology (2015), EMBO Journal (2017),Nat Commun (2019)】。在分子水平上揭示了CAPS-1和Mun13-1蛋白在胞吐分泌过程中功能关系,提出在胞吐分泌过程中CAPS-1蛋白主要依赖与Munc13-1和SNARE蛋白时序性的协同互作来完整的发挥功能。文章阐明了CAPS与Munc13家族蛋白在SNARE蛋白介导的突触分泌中的分子机制和其在突触分泌中的重要作用。
生命学院马聪教授为本文章的通讯作者,生命学院突触分泌实验室的博士生周浩,魏子晴为本文章的共同第一作者。生命学院突触分泌实验室的博士生王申,中科院上海细胞与生物化学研究所张荣光研究员和姚德强老师参与了此项工作。该成果受到国家重点基础研究项目,国家自然科学基金创新群体基金项目和面上项目,以及8797威尼斯老品牌学术前沿青年团队项目等的资助和支持。马聪教授研究团队期待更多有志于从事神经生物学和生物物理学研究的优秀青年学生以及博士后的加入。
供稿单位:马聪教授团队突触分泌实验室
Professor Cong Ma's Research Group Published New Results of CAPS and Munc13 Family Proteins Regulating Synaptic Secretion in "Cell Reports"
Learning and memory of the brain is a highly coordinated and delicate process. Synaptic vesicles carrying chemical substances such as neurotransmitters transmit synaptic information from one neuron to the next, thus completing information exchange between neurons. The mechanism of membrane fusion and related macromolecular regulation during synaptic exocytosis is a hot topic of neuroscience in recent years.
Membrane fusion in synaptic exocytosis strictly depends on the SNARE proteins (Syntaxin-1, SNAP-25 and Synaptobrevin-2), which assemble into trans-SNARE complexes in the form of " zippers" from the N terminal to the C terminal, thus bringing synaptic vesicles and plasma membrane into close proximity to complete membrane fusion. This process is highly regulated by presynaptic macromolecules or macromolecular complexes. CAPS (Calcium-dependent secretion activator) and Munc13 family proteins play important regulatory roles in the exocytosis of synaptic vesicle (SV) and dense core vesicle (DCV). Studies have shown that Munc13-1 can activate Syntaxin-1 through the "MUN" domain, thus initiating the assembly of the SNARE complexes and promoting synaptic exocytosis. Whereas the mechanism of CAPS-1 protein in synaptic exocytosis remains unclear.
On March 19st, "Cell Reports" published a research paper entitled " Structural and Functional Analysis of the CAPS SNARE-Binding Domain Required for SNARE Complex Formation and Exocytosis " by professor Cong Ma’s research group in college of life science and technology of Huazhong University of Science and Technology. This paper reports that CAPS-1 and Munc13-1 regulate synaptic exocytosis in a sequential manner by cooperatively regulating SNARE complex assembly.
The study found that the DAMH domain of CAPS-1 protein (which has structural similarity with Munc13-1 MUN domain) does not have the function of activating Syntaxin-1. On the contrary, it strongly inhibits the ability of Munc13-1 to activate Syntaxin-1 through binding with Munc13-1 MUN domian, which can effectively control the activity of Munc13-1 at the initial stage of synaptic exocytosis and ensure the effective enrichment of synaptic vesicles in the presynaptic active-zone. After Syntaxin-1 is activated, CAPS-1 stabilizes the active state of Syntaxin-1, thus accelerating the assembly of the SNARE complex and finally promoting synaptic exocytosis .

This paper expand the view of professor Cong Ma's research group in the field of neurotransmitter secretion mechanism [Science (2013), Nature Structural & Molecular Biology (2015), The EMBO Journal (2017), Nature Communications (2019)]. The results have uncovered the functional relationship between CAPS-1 and Munc13-1 in synaptic exocytosis and raised a hypothesis that CAPS-1 regulates the SNARE complex formation cooperatively with Munc13-1 in temporal and spatial level. In conclusion, these results have demonstrated the vital function and molecular mechanism of CAPS-1 and Munc13-1 in SNARE complex formation and synaptic exocytosis.
Professor Cong Ma from Huazhong University of Science and Technology is the corresponding author of this paper, the PhD candidates Hao Zhou and Ziqing Wei from the Synaptic Secretion Laboratory of School of Life Science and Technology are the co-first authors of this paper. The PhD candidate Shen Wang from Synaptic Secretion Laboratory of School of Life Science and Technology, the research fellow Rongguang Zhang and Deqiang Yao from Shanghai Institute of Cell and Biochemistry of Chinese Academy of Sciences participated in the work. This work was supported by the grants from the National Natural Science Foundation of China, the National Key Basic Research Program of China, and funds from Huazhong University of Science and Technology. Outstanding graduate students and postdocs who are interested in neuroscience and biophysics are always welcomed to join the Cong Ma’s group.
Correspondents: Hao Zhou, Ziqing Wei
Contributor: Synaptic Secretion Laboratory of Professor Cong Ma's Group