On July 22, a research paper entitled “Radiofrequency-Responsive Dual-Valent Gold Nanoclusters for Enhancing Synergistic Therapy of Tumor Ablation and Artery Embolization ” has been published in Nano Today (IF=16.907), which was performed by Prof. Xiangliang Yang and Prof. Yanbing Zhao’s group from National Engineering Research Center for Nanomedicine, College of Life Science and Technology, Huazhong University of Science and Technology.
Transcatheter Artery Embolization (TAE) and tumor ablation, represented by Radio-Frequency Ablation (RFA), are the preferred methods for unresectable advanced liver cancer. TAE-RFA combination therapy has been widely used in comprehensive treatment of hepatocellular carcinoma (HCC) through complementary and synergistic methods. For example, RFA uses high-frequency alternating current to oscillate a variety of ions in the tissue, and heat to cause tumor ablation and necrosis. However, Hepatocellular carcinoma with abundant blood supply, especially the tumor tissue far away from the electrode needle, the efficiency of radiofrequency heat deposition is extremely low through the heat dissipation of the blood stream, and the temperature gradient drops rapidly to 50 oC (the critical temperature for tumor ablation) the following. Clinically, TAE is used to close the blood supply, which can improve the "heat deposition effect" of tumor tissue to a certain extent and improve the heat distribution of tumor. How to further enhance the synergistic effect of TAE and RFA and improve the therapeutic effect of the two is still a huge challenge in the comprehensive interventional treatment of hepatocellular carcinoma.
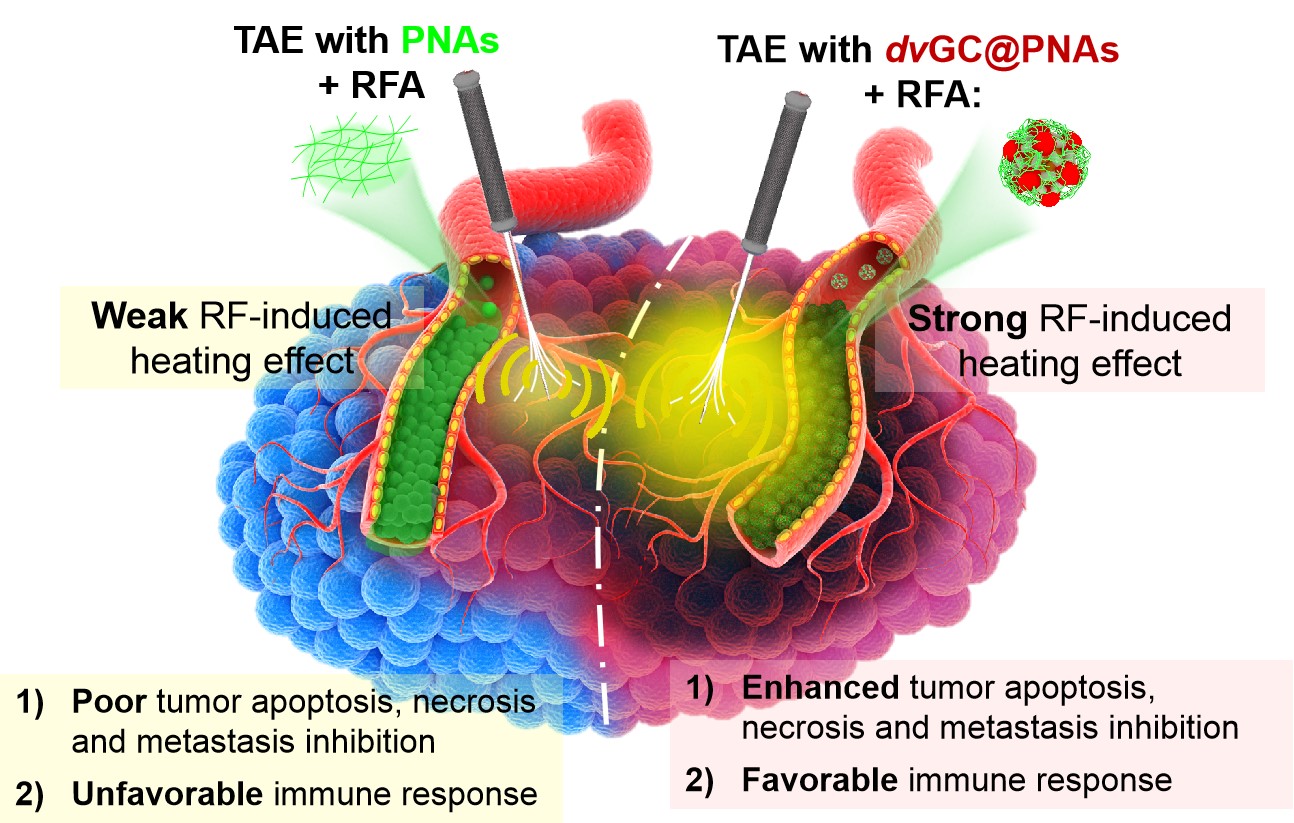
This study synthesized gold nanoclusters dvGCs with dual valence states (Au(I) ions and Au(0) atoms), and used temperature-sensitive polymer PNA to modify the surface to obtain a composite with radiofrequency thermal response. Nanogel vascular embolization agent (dvGC@PNAs). Through the temperature-sensitive sol-gel phase transition, dvGC@PNAs solves the "flow-embolization dilemma" faced by vascular embolization materials: it is in the super-liquefied catheter passability and "casting" full-level vascular embolization. dvGC@PNAs can solve the "flow embolism dilemma" faced by vascular embolic materials through thermosensitive sol-gel phase transition, that is, the contradiction between the trafficability of super liquefiable catheter and the full-scale embolism of "casting" type. Moreover, the good radiofrequency thermal effect of dvGCs combined with the vascular sealing effect of TAE can effectively enhance the thermal deposition efficiency around tumor and rich blood supply area, and improve the synergistic effect of tumor ablation and vascular embolization.
At the same time, the study also found that, dvGC@PNAs It can significantly improve the hypoxic microenvironment after embolization, activate the tumor immune response, effectively kill the remaining liver cancer cells, and effectively prevent tumor recurrence and metastasis. This study provides a new idea for the design and development of multifunctional nano materials for enhancing the efficacy of comprehensive interventional therapy for liver cancer. At the same time, the study also found that dvGC@PNAs significantly improved the hypoxic microenvironment after embolization, activated tumor immune response, killed remaining liver cancer cells and prevented tumor recurrence and metastasis effectively. This research provides new ideas for the design and development of multifunctional nanomaterials for enhancing the efficacy of comprehensive interventional therapy for liver cancer.
Prof. Xiangliang Yang and Prof. Yanbing Zhao from Huazhong University of Science and Technology, and Prof. Zheng Chuansheng from the Affiliated Union Hospital are the co-corresponding authors. Dr. Li Ling and Master Peng Xiaole from Huazhong University of Science and Technology, and Dr. Guo Xiaopeng from Affiliated Union Hospital are the co-first authors. Huazhong University of Science and Technology is the first author unit.The research was supported by the National Key Research and development Program and the National Natural Science Foundation of China.