On August 18, 2020, Professor Jianfeng Liu, leader of International Research Center for Sensory Biology and Technology of MOST and Key Laboratory of Molecular Biophysics of MOE published an article named “Illuminating the allosteric modulation of the calcium sensing receptor” on the journal Proceedings of the National Academy of Sciences of the United States of America (PNAS). In this study, they revealed the allosteric modulation of the calcium-sensing receptor.
Cells have to constantly adapt to their environment, and, as such sense, through specific receptors, a large number of nutrients, such as ions, L-amino acids (L-AAs), glucose and lipids. G protein-coupled receptors (GPCRs) form the largest family of membrane receptors and the major drug targets. Many of them are activated or modulated directly by nutrients. Among GPCRs, the calcium-sensing receptor (CaSR) is a prototypical nutrients sensory receptor activated or modulated by both calcium and amino acids, but also by endogenous and exogenous compounds such as different cations, polyamines, polypeptides and aminoglycoside antibiotics. CaSR is essential in the parathyroid gland for maintaining the extracellular calcium homeostasis. It is also expressed in other tissues such as bone, gut, kidney, and brain, where it has additional effects. Many genetic mutations that lead to loss- or gain-of-function of the CaSR, have been identified in patients with metabolic syndromes. In addition, CaSR autoantibodies that modify the signaling properties of the receptor have been identified in rare diseases. Drugs targeting CaSR are still limited as a few positive allosteric modulators, which are not enough to treat related diseases.
CaSR belongs to the class C GPCRs, most of which are activated by L-AAs or derivatives that bind to the conserved extracellular Venus flytrap (VFT) binding domain, such as the mGlu, GABAB and taste receptors. Although two teams have isolated the crystal structures of the extracellular domain of CaSR, how the binding of these nutrients in this domain triggers receptor activation remains elusive.
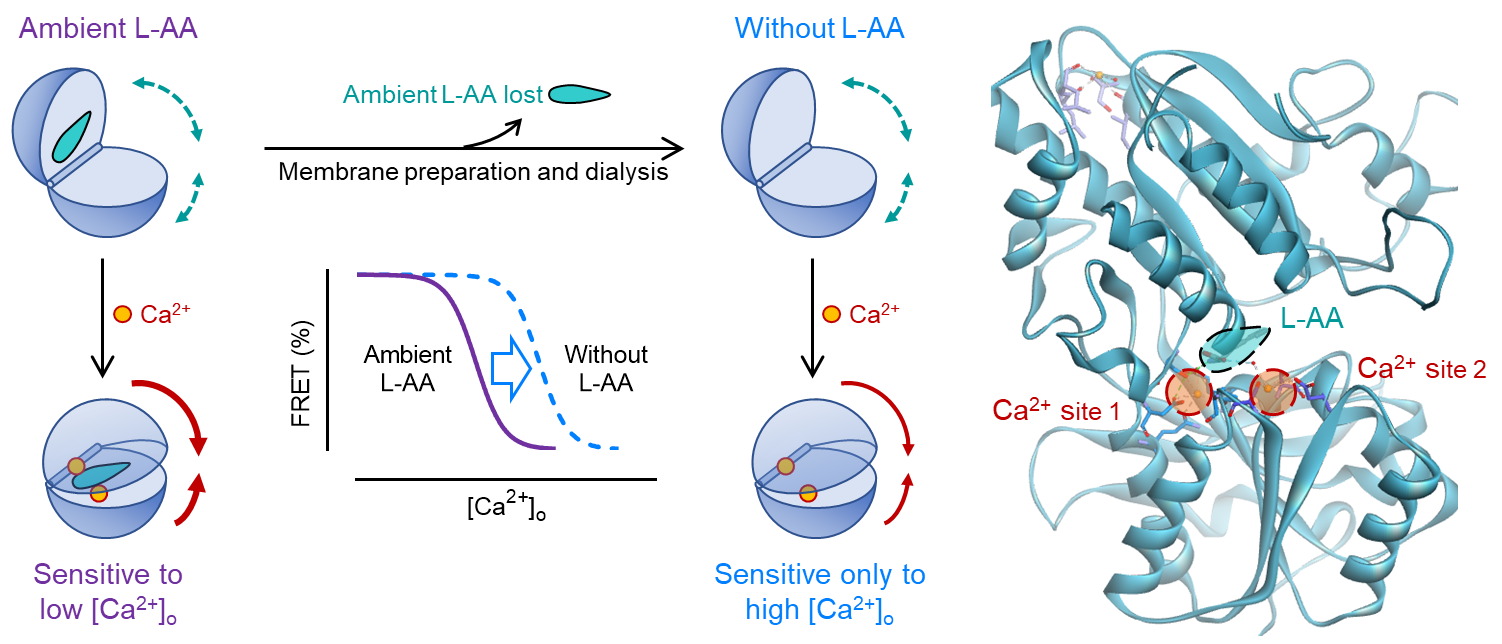
Figure: Activation of CaSR triggered upon the cooperation of calcium ions and amino acids
In this study, they first set up a time-resolved FRET conformational CaSR biosensor that could reflect the activation of the receptor. Since the sensor is independent on the downstream signaling modules, this method could be adopted to apply on the extracted membranes to get rid of the effects brought by nutrients in cellular environments. In this well-controlled condition, they revealed calcium ions by themselves could directly activate the receptor, while amino acids could potentiality this progress as positive allosteric modulators. These findings were further proved by functional assays. The cooperation between cations, anions and amino acids provide the sensibility of CaSR to the calcium concentration change within a small range, to ensure the normal physiology function of the receptor. The methods and strategy to avoid the distraction from nutrients in the environment used in this study could be applied on other receptors. This study brought supplements and improvements to the activation mechanism of CaSR, which could be useful to better understand related diseases resulted from genetic mutations. Moreover, new drugs targeting this receptor could be developed according to these findings.
This work was accomplished by team members of Prof. Jianfeng Liu’s lab. He and Prof. Jean-Philippe Pin from Institut de Génomique Fonctionnelle (France) are the co-corresponding authors of this article. This work is supported by the National Natural Science Foundation of China and the China Scholarship Council.