News Network newsletter(correspondent, Feng Chongning) Nov.2nd, team of Shangbang Gao from the School of Life at Huazhong University of Science and Technology collaborated with Prof. Shuzo Sugita team from the University of Toronto in Canada and Prof. Josep Rizo from the University of Texas Southwestern Medical Center in the United States published a research paper titled “Open syntaxin overcomes synaptic transmission defects in diverse C. elegans exocytosis mutants” in Nature Communication, an international leading journal.
Link to the paper: https://doi.org/10.1038/s41467-020-19178-x
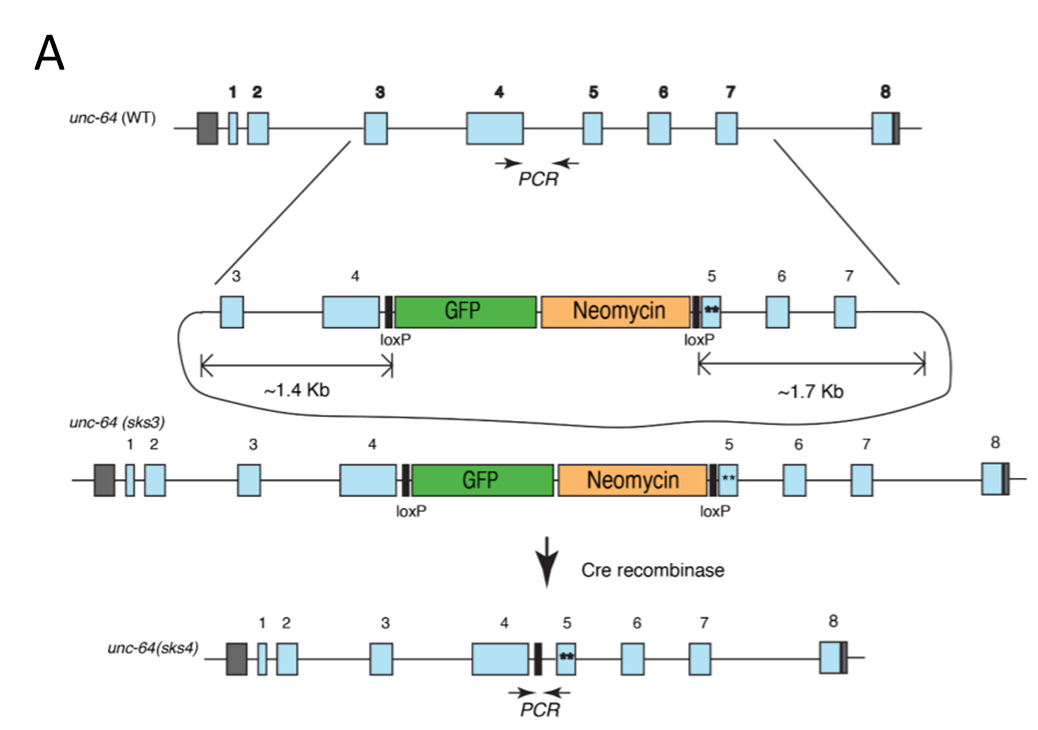
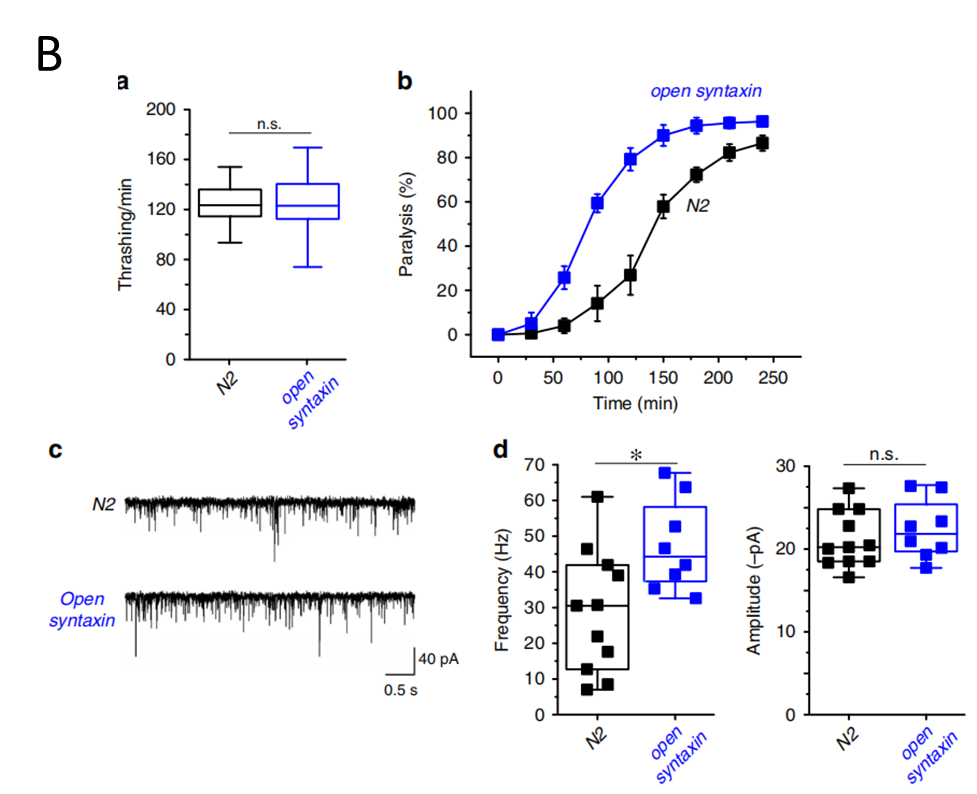
Fig. A Schematic diagram of UNC-64 / Syntaxin construction of open conformation; Fig. B The knock-in open syntaxin mutation enhances exocytosis on a wild-type background.
Synaptic vesicle exocytosis provides the chemical basis for neuronal communication, enabling the amazing diversity of functions of the brain. Soluble NSF attachment protein receptor (SNARE), composed of syntaxin, synaptobrevin /VAMP, and SNAP-25, plays a central role in neurotransmitter secretion. Compact SNARE complexes are assembled and formed to mediate the fusion of synaptic vesicles and plasma membranes, promoting the release of neurotransmitters.
Syntaxin/UNC-64 is a key SNARE protein that is crucial for exocytosis. At resting state, Syntaxin is in a closed conformation and tightly binds to and locked by Munc-18 (Syntaxin binding protein). When synaptic excitation happened, assembly of the SNARE complex requires opening the "closed" conformation of Syntaxin, called Open Syntaxin. In other words, syntaxin, an open conformation, is a crucial step in complex assembly. In nematode Caenorhabditis elegans, over-expression of a multi-copy constitutively open form of syntaxin/UNC-64 partially restored motility and strongly rescued synaptic vesicle exocytosis defects of unc-13 null worms. The rescue was suggested to be due to a specific genetic interaction between UNC-13 and syntaxin (Richmond et al., 2001). However, the strong multi-copy overexpression may introduce artificial effects, and how open Syntaxin interacts with other critical synaptic vesicle-secreted regulatory proteins is also not well known.
In this study, researchers generated the first in vivo Open Syntaxin (L166A/E167A) knock in (KI) C. elegans animal model, by using CRISPR-Cas9 technology, in UNC-64 deletion mutant. The expression level of Syntaxin in this KI animal is identical as that of wild-type nematode, so the mechanism of action of open conformation Syntaxin under physiological conditions could be well simulated. Within the Open Syntaxin animals, the researchers re-evaluated the effects of the open form of syntaxin. They introduced the unc-64(LE)/syntaxin KI into various exocytosis mutant backgrounds to systematically examine the genetic interactions between open syntaxin and other regulators of exocytosis. The results suggest that the open syntaxin KI enhances neurotransmitter release and can partially rescue the phenotypes of a wide variety of exocytosis mutants except for Munc18/UNC-18, including snt-1/synaptotagmin, unc-2/P/Q/N-type Ca2+ channel alpha-subunit and unc-31/CAPS, in addition to unc-13/Munc13 and unc-10/RIM, and enhanced exocytosis in tom-1/Tomosyn mutants. These results support the proposal that increasing the number of assembled SNARE complexes leads to enhanced neurotransmitter release. The study denotes the hypothesis of interactions between open syntaxin and UNC-13, as well as several other secretory regulatory proteins, suggesting that controlling the opening of syntaxin provides a general avenue to modulate synaptic transmission, which may help to develop therapeutic strategies for neurological diseases with impaired synaptic function.
Chi-wei Tien, PhD, university of Toronto, Canada, and Yu Bin, PhD, School of Life Sciences, Huazhong University of Science and Technology, China, are co-first authors of the paper. Prof. Shuzo Sugita from the University of Toronto, Prof. Shangbang Gao from the Huazhong University of Sci. and Tech. and Prof. Josep Rizo from the University of Texas are co-corresponding authors of this paper. The domestic work of this project is supported by the Natural Science Foundation of China, Hubei Outstanding Youth Project and Huazhong University of Science and Technology.