(Correspondent Zhang Huazhi) on December 8, Professor Wang Chenhui and Professor Rong Yueguang of Tongji Medical College of Huazhong University of science and technology published the article " RNF186 regulates EFNB1 (ephrin B1)-EPHB2-induced autophagy in the colonic epithelial cells for the maintenance of intestinal homeostasis " on Autophagy journal.

Link: https://www.tandfonline.com/doi/full/10.1080/15548627.2020.1851496
Inflammatory bowel diseases (IBD) include Crohn's disease (CD) and ulcerative colitis (UC). The main characteristics of IBD are chronic recurrent intestinal inflammation. The main clinical manifestations are recurrent abdominal pain, diarrhea and mucus pus bloody stool. The pathogenesis of IBD has not been fully understood, but more and more evidence shows that a variety of genetic, environmental and immune factors lead to the occurrence and development of IBD. The long-term risk of colorectal cancer is increased. At present, there is no effective treatment for inflammatory bowel disease, which causes great pain to patients.
Autophagy plays an important role in the regulation of cell development, differentiation, survival and senescence. Genome wide association analysis (GWAS) has identified a number of genes related to IBD susceptibility. Among them, proteins encoded by many genes are involved in the regulation of autophagy, including Atg16L1 (autophagy related 16 like 1), ulk1 (uc-51 like autophagy activating kinase 1) and IRGM (immunity related GTPase m). These findings suggest that autophagy plays an important role in host defense against pathogens and in maintaining intestinal homeostasis.
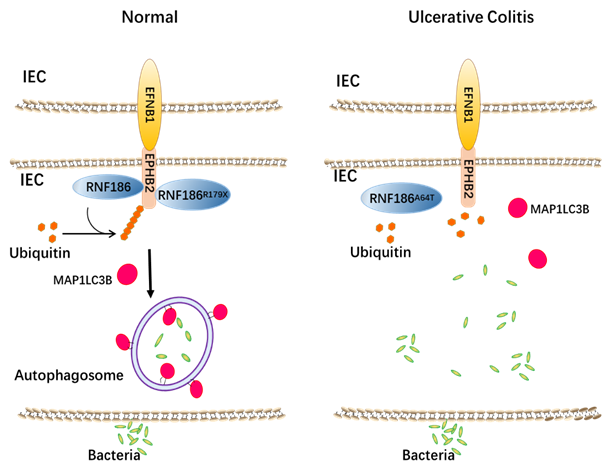
Rnf186 (ring finger protein 186) is an E3 ubiquitin ligase which containing ring-finger domain. Previous genetic studies have found that two rare variants of rnf186, RNF186A64T and RNF 186R179X, are associated with the susceptibility of ulcerative colitis. Interestingly, these two variants of RNF186 led to different susceptibility to UC. Among them, RNF186A64T led to UC susceptibility, while RNF186R179X was a protective variant. However, the role of rnf186 in ulcerative colitis and the molecular mechanism of its variation to the susceptibility of UC are not clear.
We found that RNF186 regulates EFNB1-EPHB2 signaling through ubiquitination, which is essential for EFNB 1- EPHB 2 induced autophagy and intestinal homeostasis in colon epithelial cells. In addition, we found that the recombinant protein EFNB1-FC has a certain therapeutic effect on DSS-induced colitis model in mice, suggesting that this recombinant protein has the potential to be a drug for the treatment of human ulcerative colitis. In the molecular mechanism study, we found that the receptor tyrosine kinase EphB2 is the ubiquitination substrate of RNF186, and RNF 186 can ubiquitinate EphB2 in vitro and in vivo. By mass spectrometry, we found that the k892 site of EphB2 was ubiquitinated with the catalysis of RNF186. In addition, we also found that the UC susceptible RNF186A64T lost the interaction with EphB2 and damaged the ubiquitination of EphB2, while the UC protective variant RNF186R179X increased the interaction with EphB2. These results suggest that the role of RNF186 in regulating the susceptibility of ulcerative colitis is realized through EphB2. RNF186A64T variant is a loss of function mutation, while RNF186R179X is a gain of function variation. We further found that efnb1, the ligand of EphB2, can induce autophagy in intestinal epithelial cells, which is dependent on RNF186 mediated ubiquitination of lysine at position 892 of EphB2. Through functional studies, we found that autophagy induced by EFNB1-RNF186-EPHB2 axis is essential for intestinal epithelium to clear intracellular bacterial infection. Inactivation of this pathway leadS to intestinal epithelial cells' defect in clearing intracellular bacteria, suggesting that this mechanism is the main reason for the susceptibility of UC caused by RNF186 mutation. We demonstrated the important role of rnf186 and EphB2 knockout mice in ulcerative colitis model. More importantly, we found that injection of EFNB1 FC recombinant protein into mice could activate colonic epithelial autophagy and alleviate DSS induced colitis symptoms to some extent. It is suggested that EFNB1-FC recombinant protein has the potential to be a drug for the treatment of human ulcerative colitis. In conclusion, this study found that RNF186 affects the occurrence and development of ulcerative colitis by regulating autophagy induced by EFNB1-EPHB2 pathway, and proposes that recombinant protein EFNB1-FC can be a potential drug for the treatment of human Ulcerative Colitis, and has important physiological and pathological significance.
Zhang Huazhi and Cui Zhizhi, Ph.D. candidates from the school of life, Huazhong University of science and technology, are the co-first authors of the paper. Wang Chenhui, Professor of Life College of Huazhong University of science and technology, is the corresponding author, and Rong Yueguang, Professor of Tongji Medical College of Huazhong University of science and technology, is the co-corresponding author. This research is supported by National Natural Science Foundation of China and independent innovation grant of Huazhong University of science and technology.